We need you now more than ever to provide the funds for the breakthrough research and innovative science that result in preventions, treatments, and cures. Will you join us?
“Because of the tremendous progress made, thanks in part to Foundation funding, we are really on the cusp of restoring vision for so many people.” — Michael Young, PhD, Director, Minda de Gunzburg Center for Retinal Regeneration
When you give, we are one step closer to no more parents being told their children will become blind. No more young adults being told they will go blind and no more seniors being told their final years will be in the dark.
Now is the time to make your gift and be part of the winning team that will help millions of people. The question is no longer “will we win?” It’s “how fast will we win?” That’s why the Foundation’s Board of Directors has launched Victory for Vision, a campaign to raise $75 million to accelerate the science.
A Timeline of Triumphs

1971
The Retinitis Pigmentosa Foundation is founded, which later would become the Foundation Fighting Blindness.

1974
The first Foundation-funded research center is established at the Berman-Gund Laboratory at Harvard University.

1975
The first scientific workshop on retinal degenerative diseases is held.

1979
The Foundation funds five research centers.

1984
The Foundation funds 11 research centers.

1988
The first success of retinal cell transplantation in animals is achieved.

1989
The first retinitis pigmentosa (RP) gene is identified.

1993
Vitamin A is shown to slow vision loss in RP.

1995
The Retinitis Pigmentosa Foundation’s name is changed to the Foundation Fighting Blindness.

1997
The first Stargardt disease gene (ABCA4) is identified.
1998
The Usher 2A gene is identified.

2000
An artificial retina is implanted in humans. Gene therapy restores vision in dogs with Leber congenital amaurosis (LCA).

2001
More than 100 genes are known to cause retinal degenerative diseases.

2002
Encapsulated Cell Technology (ECT) delivers CNTF (protein) to preserve vision in animals with RP.

2003
Phase 1 human clinical trial for ECT-CNTF therapy to treat RP begins.

2004
Researchers develop an Usher 1C animal model. The Foundation creates the National Neurovision Research Institute (NNRI).

2005
The CFH gene is implicated in 50 percent of age-related macular degeneration (AMD) cases.
2006
Researchers turn stem cells into retinal cells.
2006
Lucentis™ is approved by the FDA for wet AMD, halting vision loss in 90 percent of patients who are treated.
2006
Researchers use gene therapy to successfully treat Usher 1B in animal models.

2007
Phase 1 clinical trials of gene therapy for LCA begin. Phase 2 human clinical trial of DHA begins for X-linked RP.
2008
More than 140 genes are linked to retinal degenerative diseases.
2008
The Foundation establishes a partnership with Genable to develop gene therapy for dominant RP.

2009
Twenty-five people who were nearly blind experience some vision restoration in three Phase 1 clinical trials of gene therapy for LCA.
2010
Researchers use cell-based treatment to preserve vision in Usher 2A animal model.
2011
First patient treated in clinical trial of gene therapy for X-linked choroideremia.

2011
ACT begins clinical trials of stem cell treatment for Stargardt disease and dry AMD.

2012
ACT stem-cell clinical trial reports two patients demonstrated improved vision.

2013
Since its inception, VisionWalk raises almost $31 million to fund sight-saving research.

2014
The Foundation launches My Retina Tracker,™ an online retinal-disease patient registry.

2015
Spark Therapeutics launches choroideremia gene therapy clinical trial.
2016
Approximately two dozen RP participants receive stem-cell therapy in jCyte clinical trial.
2016
First RP patient receives stem-cell therapy in ReNeuron.
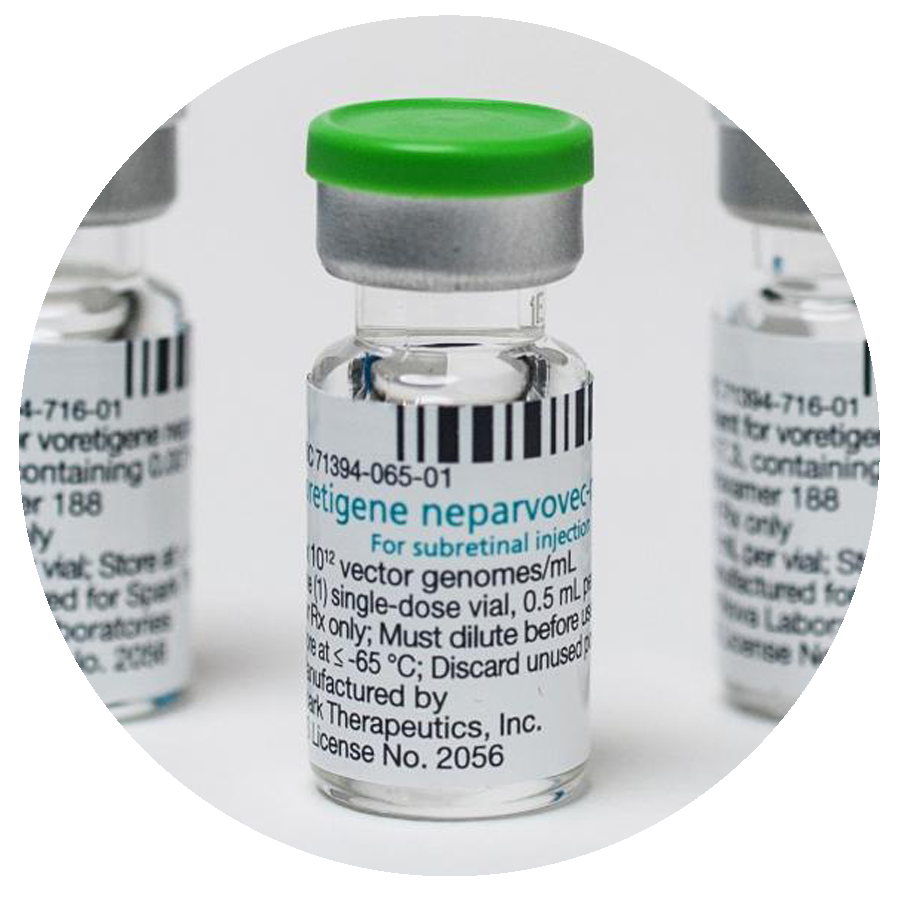
2017
FDA approves LUXTURNA (RPE65) gene therapy — first FDA-approved gene therapy for the eye or an inherited condition.

2018
The Foundation invests up to $7.5 Million in ProQR’s emerging therapy for USH2A.

2018
Nightstar’s choroideremia gene therapy moves into Phase 3 clinical trial.
2019
First Patient Receives ProQR’s Emerging LCA10 Therapy in Phase 2/3 Clinical Trial.
2020
AGTC Reports Positive Six-Month Results for XLRP Phase 1/2 Gene Therapy Trial.







